Ergosterol
Ergosterol Specification
| Appearance | White crystalline powder | Standard Packing | 100g/bag or per customer request | |
| Melting range | 158° -164° | Inventory | Normally we have Ergosterol in stock | |
| Specific rotation | > -120° | |||
| Loss on drying | <3% | Ergosterol physical parameters | ||
| Assay | ≥98% | CAS No: | 57-87-4 | |
| Formula | 396.65 | |||
| Molecular Weight | C28H44O | |||
| Synonym | Ergosterol; Provitamin D2; (22E)-Ergosta-5,7,22-trien-3-Beta-ol | |||
| Structure | 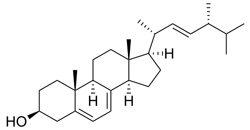 |
|||
|
Ergosterol Biosynthesis:
|
||||
| Certificate of Analysis | Ergosterol COA | |||
| Literature | Ergosterol literature | |||
| MSDS | Ergosterol MSDS | |||
| References: | ||||
| 1. | Kelly, S.L., Lamb, D.C., Corran, A.J., Baldwin, B.C., Parks, L.W. and Kelly, D.E. (1995) Purification and reconstitution of activity of Saccharomyces cerevisiae P450 61, a sterol 22-desaturase. FEBS Lett. 377, 217-220. | |||
| 2. | Yoshida, Y. and Aoyama, Y. (1991) Cytochromes P-450 in the ergosterol biosynthesis. In Ruckpaul, K. and Rein, H., Eds. Frontiers in Biotransformation, vol. 4: Microbial and Plant Cytochromes P-450: Biochemical Characteristics, Genetic Engineering and Practical Implications. Taylor & Francis, London, pp. 127-148. | |||
| . | ||||
|
Copyright © 2020 - . All Rights Reserved |
||||